Physical Properties of Alcohols
Physical Properties of Alcohols: Overview
This topic covers concepts, such as, Physical Properties of Alcohols, Water Solubility of Alcohols & Boiling Point of Alcohols etc.
Important Questions on Physical Properties of Alcohols
Alcohol which is most soluble in water is
The boiling points of alcohols decreases with increase in branching of the alkyl chain.
Ethanol has higher boiling point than ethane.
Arrange the following compounds in order of their increasing boiling points n-butyl alcohol, glycerol, n-butane, tert-butyl alcohol, sorbitol, n-butyraldehyde, isobutyl alcohol.
Explain the following :
Methanol is more soluble in water than Propan--ol.Explain the following :
Propan--ol has higher boiling point than n-Butane.
Alcohols are soluble in water whereas ethyl ether is not. Explain.
How many of the following compounds will have higher boiling point than butanol?
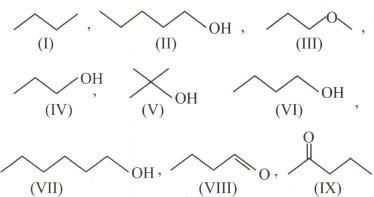
.
What is the correct order of extent of intermolecular H-bonding for primary (), secondary () and tertiary () alcohols?
Which among the following is/are true about properties of monohydric alcohols?
Which are correct statement
The boiling points of isomeric alcohols follow the order
Power alcohol is:
Which of the following statement is not correctly showing the trend of the properties mentioned?
Arrange the following compounds in increasing order of boiling points.
Methanol and ethanol are miscible in water due to
By means of calcium chloride which of following can be dried
Which of the following is not characteristic of alcohols
The compound with the highest boiling point is
Which of the following is the most suitable method for removing the traces of water from ethanol
